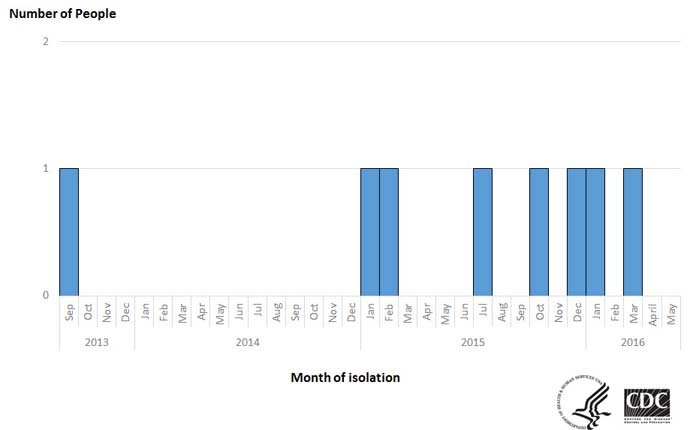
At least 8 people in 3 states have been sickened in an outbreak of Listeria infections (listeriosis) that the FDA and CDC associated with eating products processed by CRF Frozen Foods, based in Pasco, Washington, according to the CDC. The states and number sickened are as follows: California (6), Maryland (1), Washington (1). Date of onset of illness ranged from September of 2013 to March of 2016.
“Epidemiologic and laboratory evidence available at this time [May 3, 2016] indicates that frozen vegetables produced by CRF Frozen Foods of Pasco, Washington and sold under various brand names are one likely source of illness in this outbreak. Investigations are ongoing to determine if food sources used to manufacture CRF Frozen Foods products could explain some of the illnesses” (CDC).
The Listeria outbreak linked to CRF Frozen Foods was identified in March of 2016, according to the CDC.
In 2014, 2015 and 2016, the FDA inspected the CRF Frozen Foods, LLC (CRF) facility located at 1825 N. Commercial Avenue, Pasco, WA 99302. Below are excerpts from the FDA inspection reports (see “Sources” below).
March 2016 Inspection
From March 14 to 17, 2016, the FDA did a “comprehensive inspection” of the CRF facility in Pasco. The information in this section is from the March 2016 FDA inspection report.
CRF was described as a “vegetable processor and repacker.” The inspection was “conducted as part of the Seattle District FY16 work plan and in accordance with Compliance Program (CP) 7303.803, Domestic Food Safety.”
FDA noted that certain observations from the September, 2015, inspection had “been corrected and were verified during the inspection: no black residue was observed on the ceilings or on water pipes, ice buildup was not observed in the freezer, and the rolling doors have been repaired” (FDA March 2016).
Review of some documents was denied:
“During this inspection, repacking of frozen corn and peeling of whole onions was observed. A walk through the firm’s production, receiving, and storage areas was conducted as well as a finished product label review. A request to review firm records was made for the following: consumer complaints, recalls, pest control, sanitation, environmental and finished product sample results, interstate shipping documents, receiving documents, and metal detection logs. The firm declined to allow us to review any of the above requested documents” (FDA March 2016).
At the conclusion of the March, 2016, inspection, a Form FDA 483, Inspectional Observations was issued to CRF and additional observations were discussed with the company (FDA March 2016):
- “food contact areas throughout several processing lines found with chipped and cracked plastic areas that are not easily cleanable”;
- “areas of exposed aggregate throughout the firm”;
- “areas of forklift damage throughout the firm walls and doorframes”;
- “black plastic used as shielding was observed torn and difficult to clean”;
- “blue disposable bags that are designed by the firm to be used for food product were found storing waste instead of using red bags which are designated for waste”;
- “a door leading directly to the outside was observed propped open”;
- “food contact belt located in close proximity to food traffic”;
- “employees observed walking into restroom with vests on that are also worn during production”; and
- “the handwashing sink near the onion line was observed not functioning properly.”
Documents involving whole, peeled onions were denied:
“A signed affidavit was obtained covering the firm’s purchase of ingredients, production, and distribution of whole peeled onions into interstate commerce. The firm declined to provide interstate documents to include with the affidavit” (FDA March 2016).
FDA obtained environmental samples to submit for Listeria testing:
“Environmental sample number INV 885866 consisted of 55 sub samples and INV 886867 consisted of 45 sub samples were collected from the firm’s processing areas and submitted for listeria testing, and sample number INV 885868, whole peel onions, was collected and submitted for listeria testing. These samples were not completed prior to the close of the inspection” (FDA March 2016).
CRF refused photography by the FDA:
“At the beginning of the inspection, the firm refused photography and I cited two court cases to the firm President, who continued to refuse. I provided the President’s name and contact information to SCSO Schuette, as the firm’s Director of Quality Assurance stated he would be the contact for legal matters. Just before conducting environmental swabbing, another attempt was made to take photographs and the firm continued to refuse, citing proprietary concerns and company policy” (FDA March 2016).
CRF was told of legal sanctions available to the FDA:
“Firm management was advised of the legal sanctions available to the FDA if the firm does not correct serious deficiencies” (FDA March 2016).
Testing for Listeria bacteria and other pathogens is conducted in-house, according to the March 2016 inspection report, and the FDA was not allowed to review the results of these tests at the inspection:
“All testing is conducted in house unless otherwise noted. After reviewing the firm’s procedures for the testing outlined below, we requested to review the firm’s results of the following tests: incoming product tests, environmental swabs, in-process production, finished product, and re-packed product. [Employee] stated she did not think she would allow us to review the test results; however, she would verify this with Mr. Rodacy, and she departed. She came back shortly after and stated Mr. Rodacy explained we were to look at “no records” because it is ‘proprietary information’. She also explained that if the firm’s customers asked to look at the results, they also would not share this information.”
In addition to other testing, “The firm swabs processing areas (food contact) for Listeria spp., E. coli O157:H7 and Salmonella,” and according to the March 2016 report, an employee of CRF Frozen Foods told the FDA inspector that finished product testing is on a “per customer request, not on a scheduled basis.”
The following Observation 1 was listed on FDA Form FDA 483 in conjunction with the March 2016 inspection:
“The materials and workmanship of equipment and utensils does not allow proper cleaning and maintenance.”
“Specifically,
- On 03/14/16, we observed a white, plastic shovel with chips and cracks near the scoop end. This shovel was stored near the production line and is used for food contact.
- On 03/14/16, we observed blue tape being used as a temporary fix to a cracked metal plate located above the [redacted] for consumer pack line [redacted]. During this inspection, product designated for export was being repacked on this line.
- On 03/16/16, we observed chipping, cracking and missing pieces of plastic in the following areas of the onion line, which, during the inspection, was producing organic [whole] peel onions, lot code: 649560000100, 03/16/16.
- The clear plastic shied separating the [redacted] was found ripped in the middle and broken and cracked on both edges.
- The plastic conveyor belt located between the [redacted] had pieces of plastic mission from at least five of the legs. The legs come into direct contact with the onions.
- Utility knives used to hand slice undesired pieces off of the onions have etched initials directly on the blades, leaving a rough surface.”
“Supporting Evidence and Relevance:
- During the inspection, Ms. Camp verified the white shovel is used during production and used as a food contact surface on a daily basis to scoop product as needed during the re-packing process. We observed chips and cracks on the scoop, leaving areas that are not easily cleanable. There is no kill step in place for pathogens on the finished product after this step.
- Tape that is not easily cleanable was being used as a temporary fix for the cracked metal plate above the [redacted] for consumer pack line [redacted] which is used on a daily basis during the re-packing process. We also observed condensation build up on this area. There is no kill step in place for pathogens on the finished product after this step.
- The plastic shield, plastic conveyor belt and utility knives leave areas that are not easily cleanable. These are direct food contact surfaces that are used on a daily basis. There is no kill step in place for pathogens on the finished product after this step.”
September 2015 Inspection
The information in this section is from the September 1, 2015, FDA inspection report of the CRF facility located at 1825 N. Commercial Avenue, Pasco, WA 99302, which was a Washington State Department of Agriculture-assigned FDA contract inspection. “The inspection was conducted as a follow up to the December 15 and 16, 2014 inspection in which a critical violation was observed. . . . During the current inspection, green beans and corn were being processed and broccoli was being re-packaged. This inspection covered the process, re-pack and storage areas in the firm and the pest control log, master sanitation log, and customer complaint procedures were reviewed” (FDA September 2015).
Repeat violations were found at this inspection:
“Repeat violations remaining from the December 2014 inspection include black in color residue on the ceiling in front of the cooling fan in the re-pack room, areas of exposed aggregate near the corn processing equipment, and red plastic used a shielding present in the re-pack room” (FDA September 2015).
New violations were observed:
“New violations observed included condensate on piping and ceiling directly above green been, ice build-up on ceiling/wall juncture in freezer closest to shipping area, mold-like residue on chlorinated water piping on corn line, dirt and debris on white hose on corn, black color residue on multiple areas of ceiling in processing rooms, multiple areas of red and black plastic shielding in poor repair, a leak in piping conveying chlorinate water on corn line, and two rolling doors are broken” (FDA September 2015).
Listeria was found in a product, and arrangements were made to dispose of the product because it had not been shipped out to customers:
“In March of 2015 the firm was notified that one of their customers discovered a product that shipped to CFR Frozen Foods was contaminated with listeria through internal testing. The entire shipment they received was still in-house at the firm. CFR Frozen Foods has segregated this product and tagged it and arrangements are being made to dispose of this product at the local landfill. The management of this firm has stated that they have had no other reports of any problems related to products that would require them to file an RFR report” (FDA September 2015).
December 2014 Inspection
On December 15 and 16, 2014, the FDA inspected the CRF Frozen Foods, LLC facility at 1825 N. Commercial Avenue, Pasco. This section has excerpts from that FDA inspection report.
As in September of 2015, this was a WSDA-assigned FDA contract inspection of the CRF facility.
Repeat violations were found (FDA December 2014):
“The previous inspection was conducted on May 13, 2013. That inspection covered the processing, repackaging and freezer and revealed shipping dock door gaps, exposed aggregate in numerous areas near corn equipment and a broom with tape and paper on the handle.”
“During the current inspection mixed vegetalbes and corn were being repackaged and onions were being topped, tailed and peeled. This inspection covered the process, repack and storage areas in the firm and the pest control log, analysis and Micro Data were observed.”
“Repeat violations include areas of exposed aggregate near the corn processing equipment. New violations observed included a critical violation related to hand wash stations with no hot or tempered water in the onion processing area. Significant violations included a hard plastic bin with soapy water in the onion processing area. Significant violations included a hard plastic bin with soapy water and dead insects under the corn deck, black residue on the ceiling above line 1 in the repack room, red plastic shielding hanging from the hand rail and underneath the tote dump in the repack room and pallet debris, shrink wrap and product residue were noted on the warehouse floor. The freezer was observed to be over capacity, the toilet room in the onion building has trash on the floor and floors are in poor repair, doors were open to the outside in the onion processing room, and the door from the onion processing room was open to the storage area in the onion building. WSDA management is reviewing the inspection results for possible corrective action due to the presence of critical violations.”
2016 CRF Product Recalls
CRF did not recall any products until April 23, 2016, when the company recalled 11 frozen vegetable products because of possible contamination with Listeria. The companyexpanded the initial recall on May 2, 2016, to include all organic and traditional frozen vegetable and fruit products processed in its Pasco, Washington facility since May 1, 2014. This outbreak involved about 358 consumer products sold under 42 separate brands.
Sources:
- CDC May 3, 2016 Listeria Outbreak Post: http://www.cdc.gov/listeria/outbreaks/frozen-vegetables-05-16/index.html
- FDA March 2016 Inspection Report: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm505545.pdf
- FDA September 2015 Inspection Report: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm505518.pdf
- FDA December 2014 Inspection Report: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm505530.pdf