Product Liability
Product Liability
Product liability news includes information about outbreaks of infection, painful implants, surgical errors and other acts of negligence. Our law firm helps people nationwide.
Product Liability
FDA Finds Elevated Lead Levels in More Cinnamon Products
Product Liability
Seizures, Death Prompt FDA Warning for Neptune’s Fix
Product Liability
Lead in Applesauce Pouches Sickens 9 in MN
Product Liability
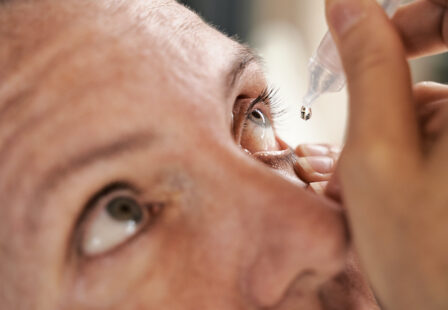
Eye Drops Recall for Vision Loss Risk at CVS, Target, Rite Aid
Product Liability
Injuries Prompt Empower Juicer Recall – Sold at Target, Walmart
Product Liability
Peloton Recall – “Immediately Stop Using” PL01 Bikes
Product Liability
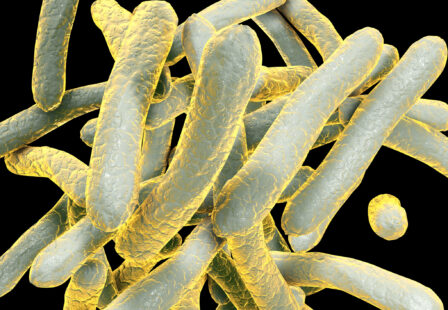
How Common are Pseudomonas aeruginosa Deaths
Product Liability
Purely Soothing Eye Drops Recalled for Blindness Risk
Product Liability
Fabuloso Multi-Purpose Cleaner Recall
Product Liability
EzriCare and Delsam Pharma Artificial Tears Recall
Product Liability