Purely Soothing Eye Drops have been recalled for bacterial contamination that can cause eye infections that could result in blindness. Consumers who have purchased this product should stop using it immediately.
Anyone who has experienced an adverse reaction can file a report with the U.S. Food and Drug Administration’s (FDA’s) MedWatch Adverse Event Reporting program either online, by regular mail, or by fax. When Pharmedica USA LLC issued the recall on March 3, 2023, it had not received any reports of adverse events or illnesses related to the product.
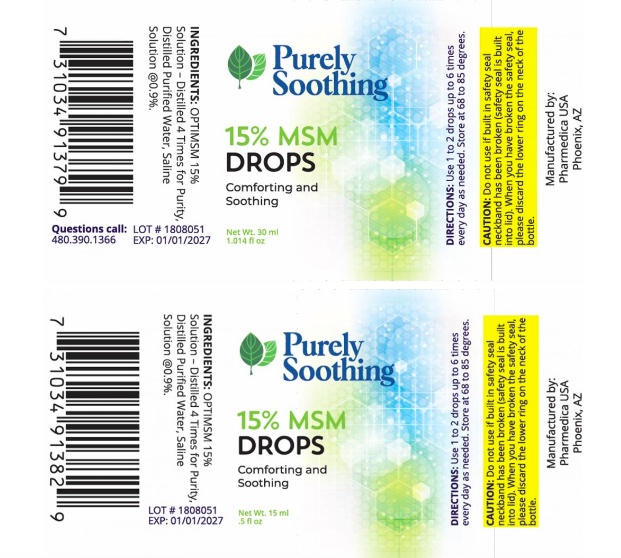
The recalled drops were sold online and at trade shows and can be identified with the following information:
- 1 oz HDPE plastic bottles
- LOT#: 2203PS01
- UPC 7 31034 91379
- 1/2-oz HDPE plastic bottles
- LOT#: 1808051
- UPC 7 31034 91382 9
Contact The Pritzker Hageman Legal Team
Phone: 888-377-8900 | Text: 612-261-0856