Product Liability
Methylprednisolone Acetate
News about meningitis from methylprednisolone acetate.
Medical Products And Procedures
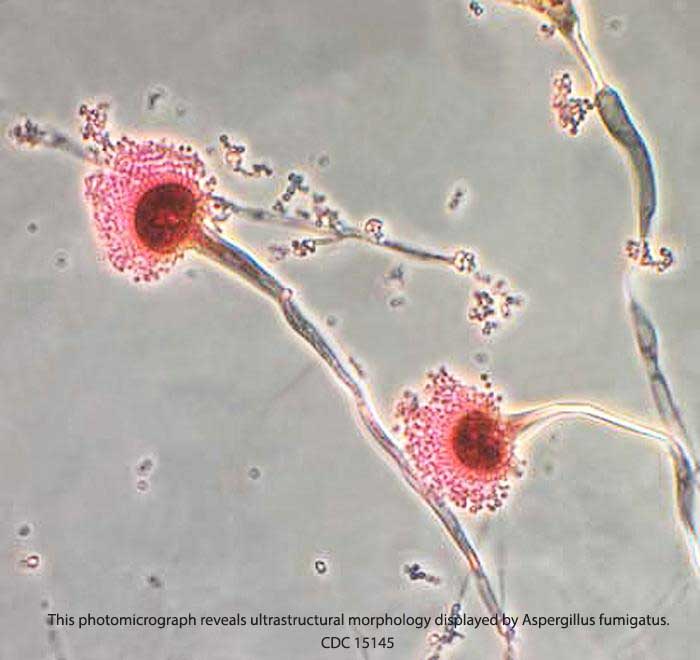
Paraspinal and Spinal Infections from Injections
Medical Products And Procedures
Lawsuits against NECC for Fungal Meningitis are Asking for Millions
Product Liability