Product Liability
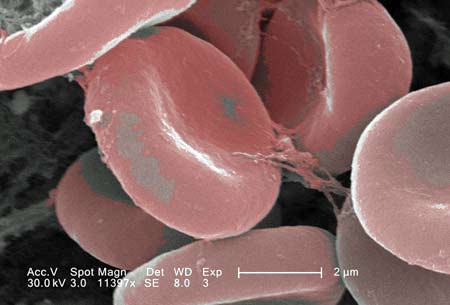
Blood Clot
Medication can cause a blood-clot, also referred to as thrombosis. When this happens the person can have a stroke, heart attack or die. Our law firm handles these claims.
Food Poisoning
Can New Test Prevent STEC-HUS Kidney Failure?
Product Liability
Hospira Recall Lawsuit: Kidney Damage, Embolism
Product Liability