At least four cardiac patients at the University of Washington Medical Center have now contracted Legionnaires’ disease, a severe form of pneumonia that can be fatal in up to 50% of hospital-acquired cases. Two of these patients have subsequently died.
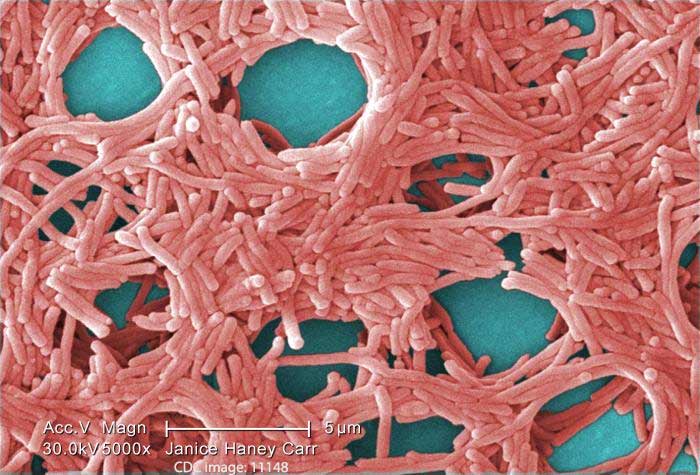
An investigation initiated by hospital officials after the diagnoses initially revealed the presence of Legionella pneumonia bacteria in an ice machine and two sinks at UW Medical Center’s Cascade Tower. Now, however, these bacteria have also been discovered in three medical devices used to heat and cool patients during heart surgery. (1) These particular heating-cooling devices were manufactured by CardioQuip, headquartered in Bryan, Texas.
Hospital Disease Transmission via Heater-Cooler Devices
As reported by The Seattle Times, this transmission of Legionnaires’ disease itself through heater-cooler devices has never been witnessed before:
“Legionnaires’ disease has not been previously proven to be transmitted in this fashion,” Tina Mankowski, a UW Medicine spokeswoman, said in a statement Monday. No direct link with patient infections has been confirmed.” (1)
In an email to The Seattle Times, Mankowski also remarked that “Exposure to water in the Cascade Tower inpatient units is highly likely to be the source of the Legionella infections (not exposure to the heater / cooler units.” (1)

However, the devices have been implicated in often-fatal hospital outbreaks of other diseases which, like Legionnaires’ disease, are transmitted through aerosolized water vapor. In an October 15, 2015 safety communication, the FDA published an alert warning the public of the danger of the devices after it received 32 Medical Device Reports (MDRs) of Nontuberculous Mycobacteria (NTM) infections in patients undergoing cardiothoracic surgical procedures:
“Heater-cooler devices are used during cardiothoracic surgeries, as well as other medical and surgical procedures to warm or cool a patient to optimize medical care and improve patient outcomes. Heater-cooler devices include water tanks that provide temperature-controlled water to external heat exchangers or warming/cooling blankets through closed circuits. Although the water in the circuits does not come into direct contact with the patient, there is the potential for contaminated water to enter other parts of the device or transmit bacteria through the air (aerosolize) through the device’s exhaust vent into the environment and to the patient.” (2)
Outbreaks of Nontuberculous Mycobacteria associated with heating-cooling devices (none of which were manufactured by CardioQuip) include one in 2015 that sickened 8 patients – 4 of whom died – at WellSpan York Hospital in central Pennsylvania. (3) The manufacturer of the devices in this outbreak, LivaNova PLC, is now being sued in a $5 million federal class-action lawsuit. Our law firm is not involved in this lawsuit.
Legionnaires’ Disease Timeframe at UW Medical Center
- August 26, 2016: A 30-year-old female cardiac patient was diagnosed with Legionnaires’ disease.
- August 27, 2016: A 50-year-old female cardiac patient died; the Legionella pneumonia bacteria was subsequently found in her body upon autopsy.
- September 6, 2016: A 50-year-old male patient was diagnosed with Legionnaire’s disease; he died on September 8th.
- The week of September 12th: a 40-year-old male patient was diagnosed with LD and is currently being treated.
UW Medical Center officials are currently contacting all high-risk patients hospitalized in the Cascade Tower between August 24th and September 13th to warn them of the dangers of Legionella exposure. They have established a UW Medicine information line for patient questions about Legionella pneumonia at 855.520.2252. Care providers have been informed to contact Dr. Estella Whimbey, associate medical director and medical director for infection control, at 206.598.0106 (office), 206.583.9096 (pager), or 206.321.5853 (cell phone).
They are also currently chemically treating the water in the Cascade Tower and installing special water filters in all sinks and showers in this facility.
People who contract Legionnaires’ disease in a hospital or other healthcare facility may have the right to sue either the facility or medical equipment manufacturers for wrongful death or personal injury. Patients and their families should contact a law firm regarding a lawsuit for compensation.
Sources:
- Aleccia, JoNel. “Operating-room machines test positive for Legionella at UW Medicine.” The Seattle Times. Web. 19 Sep. 2016.
- Press Release. “Nontuberculous Mycobacterium Infections Associated with Heater-Cooler Devices: FDA Safety Communication.” FDA. Web. 15 Oct. 2015.
- 3. Avril, Tom. “Pa. hospital reports four dead after device-related infections.” Philly.com. Web. 28 Oct. 2015.