Sagent Pharmaceuticals, Inc. issued a nationwide recall of two lots of Atracurium Besylate Injection, USP, 50mg/5mL single-dose vials (NDC 25021-659-05) and four lots of Atracurium Besylate Injection, USP, 100mg/10mL multi-dose vials (NDC 25021-672-10). All lots were manufactured by Emcure Pharmaceuticals Ltd. and distributed by Sagent.
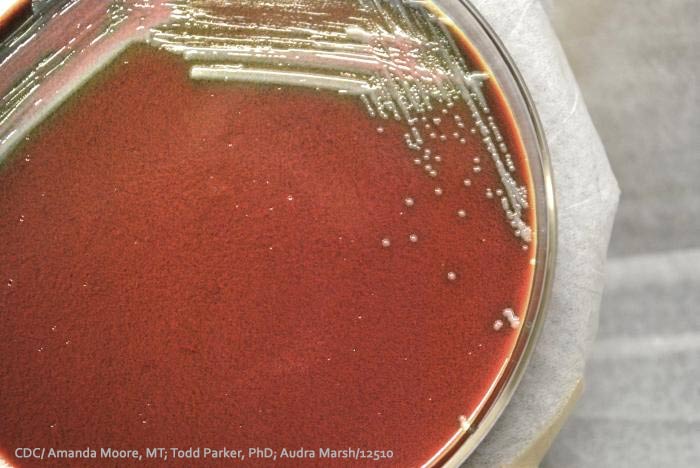
The recall was issued because FDA observations during a routine plant inspection found evidence that these lots may not be sterile. If a non-sterile drug is andministered via an intravenous (IV) injection, there is a risk of an infection that could cause severe illness or wrongful death.
Our lawyers are currently representing people who acquired infections from contaminated injections manufactured by another company. These types of infections are caused bacteria or fungi mold. Lawsuits can include product liability and medical malpractice claims.
Recall Details
The lot numbers of the recalled product are VATA012, VATA015 (50mg/5mL) and VATB012, VATB013, VATB014, VATB017 (100mg/10mL). These lots were distributed to hospitals, wholesalers and distributors nationwide from February 2014 through February 2015.
This product is used as an adjunct to general anesthesia, to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
After learning of the FDA observations at the Emcure plant, Sagent Pharmaceuticals transferred the manufacture of this product to its own facility. Atracurium Besylate Injection manufactured at the Sagent facility is not impacted by this recall.
Sagent has stated that the company is not aware of any adverse patient events resulting from the use of the subject product lots.
What to Do about an Infection from Atracurium Besylate Injection
If you or a loved one developed an infection after receiving an injection with this product, you can contact our law firm for help. Our attorneys have handled many cases like yours and have won millions for our clients, including dozens of multimillion-dollar settlements and verdicts.
If you suspect an infection from this product, you should contact your doctor or healthcare provider immediately.